Today on Drug Discovery & Development
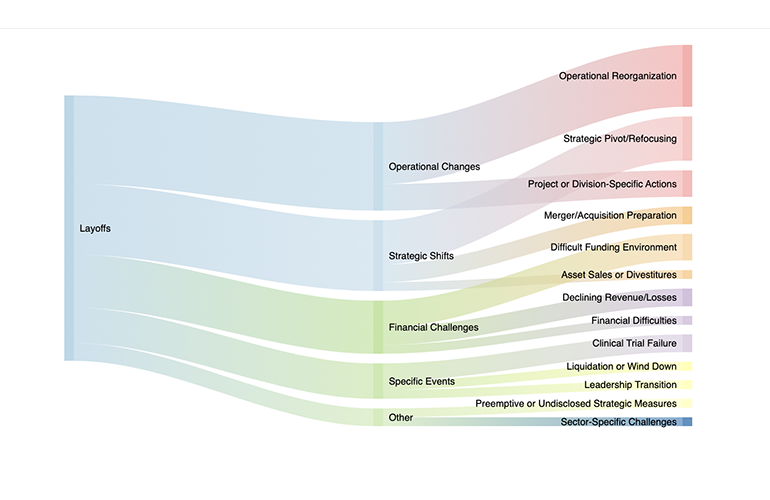
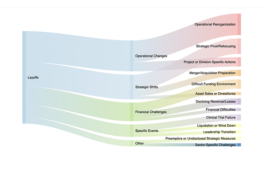
Layoff tracker: Mapping the 7,300-plus biotech and pharma layoffs in early 2024
[Updated on April 10, 2024] The biotech sector continues to experience a significant number of layoffs. Major cuts in early 2024 include companies like Pfizer, Thermo Fisher Scientific, Sanofi, Catalent, and GSK. Bayer reduced its executive team from 14 to eight members as part of a major operating overhaul. Catalent laid off 130 workers at…As biotech struggles, Big Pharma spends and trims workers
Many smaller biotech companies continue to face a cash crunch as a result of a challenging funding climate and a difficult IPO market. In the past couple of years, many smaller biotechs are running out of cash and being forced to cut jobs or shut down entirely. Median deal sizes for seed, Series A and Series B rounds…Drug Discovery and Development See More >

20 biotech startups attracted almost $3B in Q1 2024 funding
The top 20 healthcare-focused biotech companies collectively raised $2.9 billion in the first quarter of 2024, according to data sourced from Crunchbase. That represents a 161% increase compared to the $1.1 billion raised by the 20 largest funding rounds involving healthcare-focused biotech companies in Q1 2023, indicating more confident bets on the market viability of…
Sponsored Content See More >
Genomics/Proteomics See More >

Unleashing a new frontier: The power of germline clinico-genomic data to drive therapeutic development
Over the past decade, the use of deeper sources of real-world data across all stages of the drug development life cycle has become increasingly important to guide disease understanding, trial designs, clinical guidelines, regulatory submissions and post-market studies. The advent of these deeper sources was prompted by the HITECH Act, which had the effect of…

NVIDIA expands BioNeMo platform with new foundation models and microservices for AI-powered Drug Discovery

Navigating the cancer progression pathway with liquid biopsy

Microsoft and 1910 Genetics: AI-powered partnership targets billion-dollar savings and growth in drug discovery

Culmination Bio partners with Merck on disease-agnostic patient data
Infectious Disease See More >

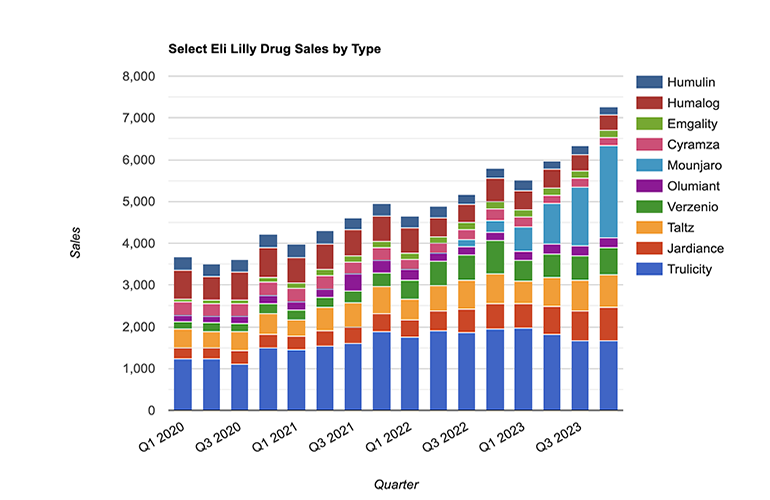
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…

Lumen Bioscience cracks the code on spirulina as a biologics factory for c. diff, metabolic disease and more

Vaccine mega-trials: Rare behemoths in the vaccine trial landscape

Biden names 31 tech hubs: Here are 10 relevant to pharma and biotech

An overview of the RSV vaccine landscape: GSK aims to extend its approval of Arexvy?
Oncology See More >

20 biotech startups attracted almost $3B in Q1 2024 funding
The top 20 healthcare-focused biotech companies collectively raised $2.9 billion in the first quarter of 2024, according to data sourced from Crunchbase. That represents a 161% increase compared to the $1.1 billion raised by the 20 largest funding rounds involving healthcare-focused biotech companies in Q1 2023, indicating more confident bets on the market viability of…