Today on Drug Discovery & Development
ten23 health to develop large-volume injectables for Ypsomed’s YpsoDose
Ypsomed announced today that it entered into a partnership with ten23 health to develop therapeutics for the YpsoDose wearable injector. Burgdorf, Switzerland-based Ypsomed designed YpsoDose for the subcutaneous self-injection of large-volume doses. ten23 health, a Swiss CDMO, offers drug development, filling and device assembly expertise to contribute to the product offering. The strategic collaboration sees…The rise of ‘Ozempic babies’ and the uncharted territory of semaglutide in pregnancy
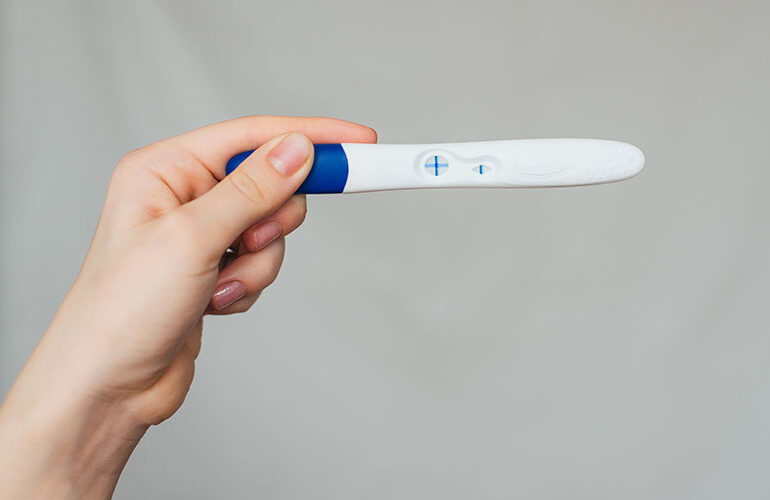
Ozempic, Rybelsus and Wegovy have transformed the diabetes and weight loss treatment landscape, but when it comes to the impact of their active ingredient, semaglutide, on fetal development, “the answer is we do not know,” said Dr. Marijane Hynes, clinical professor of medicine at the George Washington University School of Medicine and Health Sciences. Hynes…Drug Discovery and Development See More >

Medincell, AbbVie partner on long-term injectable therapies
Medincell and AbbVie today announced a collaboration to co-develop and commercialize up to six therapeutic products across several areas. The partnership spans multiple therapeutic spaces and indications. Under the agreement, Medincell will use its commercial-stage, long-acting injectable technology platform to formulate innovative therapies. It plans to conduct formulation activities and preclinical studies, including supportive CMC…
Sponsored Content See More >
Genomics/Proteomics See More >

Unleashing a new frontier: The power of germline clinico-genomic data to drive therapeutic development
Over the past decade, the use of deeper sources of real-world data across all stages of the drug development life cycle has become increasingly important to guide disease understanding, trial designs, clinical guidelines, regulatory submissions and post-market studies. The advent of these deeper sources was prompted by the HITECH Act, which had the effect of…

NVIDIA expands BioNeMo platform with new foundation models and microservices for AI-powered Drug Discovery

Navigating the cancer progression pathway with liquid biopsy

Microsoft and 1910 Genetics: AI-powered partnership targets billion-dollar savings and growth in drug discovery

Culmination Bio partners with Merck on disease-agnostic patient data
Infectious Disease See More >

Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…

Lumen Bioscience cracks the code on spirulina as a biologics factory for c. diff, metabolic disease and more

Vaccine mega-trials: Rare behemoths in the vaccine trial landscape

Biden names 31 tech hubs: Here are 10 relevant to pharma and biotech

An overview of the RSV vaccine landscape: GSK aims to extend its approval of Arexvy?
Oncology See More >

20 biotech startups attracted almost $3B in Q1 2024 funding
The top 20 healthcare-focused biotech companies collectively raised $2.9 billion in the first quarter of 2024, according to data sourced from Crunchbase. That represents a 161% increase compared to the $1.1 billion raised by the 20 largest funding rounds involving healthcare-focused biotech companies in Q1 2023, indicating more confident bets on the market viability of…