
[Adobe Stock]
While it is easy for individuals to dabble with the technology, quantifying the actual enterprise value for biopharma firms is often less clear. “We kept hearing the same question over and over again from the C-suite: What’s the value to my company? What’s the size of the prize?” said Adam Israel, managing director at Deloitte Consulting, and co-author of the report, “Realizing Transformative Value from AI & Generative AI in Life Sciences.”
The answer to the preceding question lies, to a large degree, in execution.
Why GenAI can be a transformative opportunity for biopharma
Biopharma companies that are strategic about deploying genAI could carve out a significant competitive advantage, deploying it not for isolated use cases but across multiple domains. “I think we’re going to see almost a tale of two companies emerge in the coming years,” Israel said. “On one side, there will be the companies that decided to be proactive.”
The critical play is to elevate genAI as a core strategic asset throughout the organization rather than for siloed pilots. Deloitte sees the most potential in accelerating R&D (30-45% of value) followed by commercialization (25-35% of value). Rounding out the top four are manufacturing and supply chain (15-25% of value potential) and enabling areas such as IT, HR and finance (collectively, 5-15% of value potential).
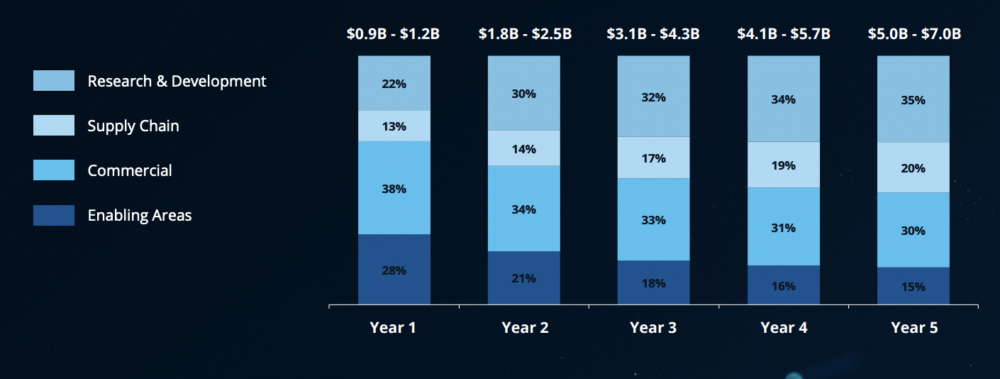
R&D and commercial sectors within biopharma could see the greatest returns from genAI. [From the Deloitte report “Realizing Transformative Value from AI & Generative AI in Life Sciences“]
Stringing together genAI use cases
In its report, Deloitte analyzed 20 comprehensive, end-to-end AI use cases spanning functions such as R&D, manufacturing, supply chain and commercial operations. Rather than viewing AI use cases in isolation, the study linked them together like “pearls on a string.” “All that really means is that we’re doing one use case at a time, but we’re looking at a domain like clinical development, and we’re looking at each use case being a pearl, and we’re trying to bring them together so that you can create more value, if you will, by stringing these capabilities, one at a time,” Israel said. That means potentially transforming entire value streams or processes like product launches and clinical development lifecycles.
From AI “book smarts” to “street smarts”
One chief differentiator between genAI-based large language models is their ability to understand a wide variety of human language contexts. Contrast with older approaches such as symbolic AI (also known as “Good Old-Fashioned AI” or GOFAI) that formed the basis of expert systems where programmers carefully created expert systems based on explicit rules and fixed logic. These older systems typically relied on a rigid set of rules and required narrowly defined parameters.Similarly, many traditional natural language processing systems could excel at processing relatively narrow types of texts from given disciplines. Such traditional systems had “book smarts,” Israel said. But more recent large language models have “street smarts,” he added. “You can put it into different types of scenarios. You don’t have to explicitly tell it what to do. It can improvise, it can maneuver.” The same models can thus be customized to work on regulatory, marketing and financial documents. “You name it,” Israel said. “Over time, we can refine it and get it more accurate so that it can do that one job really well and essentially match that same level of accuracy of traditional AI technologies, but at a fraction of the cost and time.”
Those “street smarts” stand in contrast to other types of technologies such as blockchain and traditional machine learning that were more esoteric and not that accessible to the general public. “You needed a certain level of scientific skill to use them,” Israel said. “But if you ask almost anyone next to you, they can use [a mainstream large language model] today; they’re already starting to use it.”
Democratizing AI: Accessibility for enterprises and individuals
Large language models’ accessibility also opens up the potential for enterprise users. “You can now turn all your data into insights and assets like never before,” Israel said. The specific value levers that AI and generative AI can provide are efficiency, experience, and capability, he added. Efficiency is often the low-hanging fruit — doing something better, faster, and cheaper. Automating, for instance, tedious data-entry work. Experience refers to completely transforming expectations for a given technology. Think about the evolution, for instance, of chatbots from the 2000s to present day.
“And what this is, is using AI and genAI to turn your data into assets and to essentially extract insights from things that you just didn’t have the manpower to do in the past. Because so many companies today have SharePoint sites and databases full of information. And what we’re seeing is like an iceberg effect – only a small subset of that data and information comes to light and helps you make decisions,” Israel said.
Ensuring trustworthy AI and preparing the workforce
To guarantee unbiased and fair use of AI, particularly when dealing with sensitive customer or patient data, Adam Israel emphasizes the necessity of a “trustworthy AI framework,” noting that “bad data in equals bad data out.” As organizations incorporate AI more deeply into their operations, Israel highlights the development of new leadership roles: “We’re starting to see as well the emergence of different roles at different companies where AI is in the name.”
Furthermore, he advises on the importance of training: “Our big recommendation to companies is you need to push your people through some minimum viable prompt training,” which ensures that employees can generate precise prompts that tailor AI outputs to specific needs and scenarios.
A ‘no regrets’ approach to placing technology best
Given the circumstances, Deloitte recommends that companies make “no regrets” investments in the technology. That is, to build out a single capability once and apply it “many times based on the needs of different divisions, different use cases, different customer segments – you name it,” Israel said.
The risks of taking a wait and see approach are significant, Israel said. “If you’re slow out of the gate right now, I think the risk is that there’s an opportunity cost,” he said. “The opportunity cost is if you want to wait and see, like some companies have done with traditional technologies, and then make investments 6-12 months or a year or two down the line, the risk is great because of this competitive advantage that we’re seeing right now.”
Filed Under: Data science, machine learning and AI



